The nature of chromium
Chromium, element symbol Cr, atomic number 24, relative atomic mass 51.996, belongs to the transition metal element of group VIB of the periodic table of chemical elements. Chromium metal is body-centered cubic crystal, silver-white, density 7.1g/cm³, melting point 1860℃, boiling point 2680℃, specific heat capacity at 25℃ 23.35J/(mol·K), heat of vaporization 342.1kJ/mol, thermal conductivity 91.3 W/(m·K) (0-100°C), resistivity (20°C) 13.2uΩ·cm, with good mechanical properties.
There are five valences of chromium: +2, +3, +4, +5 and +6. Under the conditions of endogenous action, chromium is generally +3 valence. Compounds with +trivalent chromium are the most stable. +Sixvalent chromium compounds, including chromium salts, have strong oxidizing properties. The ionic radii of Cr3+, AI3+ and Fe3+ are similar, so they can have a wide range of similarities. In addition, the elements that can be replaced with chromium are manganese, magnesium, nickel, cobalt, zinc, etc., so chromium is widely distributed in magnesium iron silicate minerals and accessory minerals.
Application
Chromium is one of the most widely used metals in modern industry. It is mainly used in the production of stainless steel and various alloy steels in the form of ferroalloys (such as ferrochrome). Chromium has the characteristics of hard, wear-resistant, heat-resistant and corrosion-resistant. Chrome ore is widely used in metallurgy, refractory materials, chemical industry and foundry industries.
In the metallurgical industry, chromium ore is mainly used to smelt ferrochrome and metallic chromium. Chromium is used as a steel additive to produce a variety of high-strength, corrosion-resistant, wear-resistant, high-temperature, and oxidation-resistant special steels, such as stainless steel, acid-resistant steel, heat-resistant steel, ball bearing steel, spring steel, tool steel, etc. Chromium can enhance the mechanical properties and wear resistance of steel. Metal chromium is mainly used to smelt special alloys with cobalt, nickel, tungsten and other elements. Chrome plating and chromizing can make steel, copper, aluminum and other metals form a corrosion-resistant surface, which is bright and beautiful.
In the refractory industry, chromium ore is an important refractory material used to make chrome bricks, chrome magnesia bricks, advanced refractories and other special refractory materials (chrome concrete). Chromium-based refractories mainly include bricks with chrome ore and magnesia, sintered magnesia-chrome clinker, molten magnesia-chrome bricks, molten, finely ground and then bonded magnesia-chrome bricks. They are widely used in open hearth furnaces, induction furnaces, etc. Metallurgical converter and rotary furnace lining of cement industry, etc.
In the foundry industry, chromium ore will not interact with other elements in molten steel during the pouring process, has a low thermal expansion coefficient, is resistant to metal penetration, and has better chilling performance than zircon. Chrome ore for foundry has strict requirements on chemical composition and particle size distribution.
In the chemical industry, the most direct use of chromium is to produce sodium dichromate (Na2Cr2O7·H2O) solution, and then to prepare other chromium compounds for use in industries such as pigments, textiles, electroplating, and leather making, as well as catalysts.
The finely ground chromium ore powder is a natural coloring agent in the production of glass, ceramics and glazed tiles. When sodium dichromate is used to ravage leather, the protein (collagen) and carbohydrates in the original leather react with chemical substances to form a stable complex, which becomes the basis of leather products. In the textile industry, sodium dichromate is used as a mordant in fabric dyeing, which can effectively attach dye molecules to organic compounds; it can also be used as an oxidant in the manufacture of dyes and intermediates.
Chromium mineral
There are more than 50 kinds of chromium-containing minerals that have been discovered in nature, but most of them have low chromium content and scattered distribution, which has low industrial use value. These chromium-containing minerals belong to oxides, chromates and silicates, in addition to a few hydroxides, iodates, nitrides and sulfides. Among them, chromium nitride and chromium sulfide minerals are only found in meteorites.
As a mineral species in the chromium ore subfamily, chromite is the only important industrial mineral of chromium. The theoretical chemical formula is (MgFe)Cr2O4, in which Cr2O3 content accounts for 68%, and FeO accounts for 32%. In its chemical composition, the trivalent cation is mainly Cr3+, and there are often Al3+, Fe3+ and Mg2+, Fe2+ isomorphic substitutions. In the actual produced chromite, part of Fe2+ is often replaced by Mg2+, and Cr3+ is replaced by Al3+ and Fe3+ to varying degrees. The complete degree of isomorphic substitution among the various components of chromite is not consistent. The four-order coordination cations are mainly magnesium and iron, and the complete isomorphic substitution between magnesium-iron. According to the four-division method, chromite can be divided into four subgroups: magnesium chromite, iron-magnesium chromite, mafic-iron chromite and iron-chromite. In addition, chromite often contains a small amount of manganese, A homogeneous mixture of titanium, vanadium and zinc. The structure of chromite is of the normal spinel type.
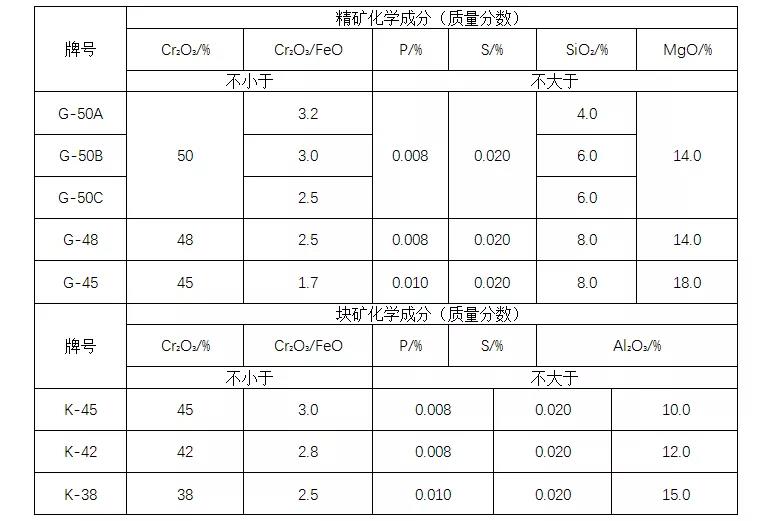
4. Quality standard of chromium concentrate
According to different processing methods (mineralization and natural ore), chromium ore for metallurgy is divided into two types: concentrate (G) and lump ore (K). See the table below.
Quality requirements for chromite ore for metallurgy
Chrome ore beneficiation technology
1) Re-election
At present, gravity separation occupies an important position in the beneficiation of chromium ore. The gravity separation method, which uses loose layering in the aqueous medium as the basic behavior, is still the main method for enriching chromium ore worldwide. The gravity separation equipment is a spiral chute and a centrifugal concentrator, and the processing particle size range is relatively wide. Generally, the density difference between chromium minerals and gangue minerals is greater than 0.8g/cm3, and the gravity separation of any particle size greater than 100um can be satisfactory. the result of. Coarse lumps (100 ~ 0.5mm) ore is sorted or pre-selected by heavy-medium beneficiation, which is a very economical beneficiation method.
2) Magnetic separation
Magnetic separation is a beneficiation method that realizes separation of minerals in a non-uniform magnetic field based on the magnetic difference of the minerals in the ore. Chromite has weak magnetic properties and can be separated by vertical ring high gradient magnetic separators, wet plate magnetic separators and other equipment. The specific magnetic susceptibility coefficients of chromium minerals produced in various chromium ore producing areas in the world are not much different, and are similar to the specific magnetic susceptibility coefficients of wolframite and wolframite produced in various regions.
There are two situations in using magnetic separation to obtain high-grade chromium concentrate: one is to remove the strong magnetic minerals (mainly magnetite) in the ore under a weak magnetic field to increase the ratio of ferrochrome, and the other is to use a strong magnetic field. Separation of gangue minerals and recovery of chromium ore (weakly magnetic minerals).
3) Electric selection
Electric separation is a method of separating chromium ore and silicate gangue minerals by using the electrical properties of minerals, such as differences in conductivity and dielectric constant.
4) Flotation
In the process of gravity separation, fine-grained (-100um) chromite ore is often discarded as tailings, but the chromite of this size still has a high utilization value, so the flotation method can be used for low-grade fine Granular chromite ore is recovered. Flotation of chromium ore with 20% ~40% Cr2O3 in tailings and serpentine, olivine, rutile and calcium magnesium carbonate minerals as gangue minerals. The ore is finely ground to 200μm, water glass, phosphate, metaphosphate, fluorosilicate, etc. are used to disperse and inhibit the sludge, and unsaturated fatty acid is used as a collector. The dispersion and suppression of gangue sludge is very important to the flotation process. Metal ions such as iron and lead can activate chromite. When the pH value of the slurry is below 6, the chromite will hardly float. In short, the flotation reagent consumption is large, the concentrate grade is unstable, and the recovery rate is low. Ca2+ and Mg2+ dissolved from gangue minerals reduce the selectivity of the flotation process.
5) Chemical beneficiation
Chemical method is to directly treat certain chromite ore that cannot be separated by physical method or the cost of physical method is relatively high. The Cr/Fe ratio of concentrate produced by chemical method is higher than that of ordinary physical method. Chemical methods include: selective leaching, oxidation reduction, melting separation, sulfuric acid and chromic acid leaching, reduction and sulfuric acid leaching, etc. The combination of physical-chemical methods and the direct treatment of chromium ore by chemical methods are one of the main trends in chromite beneficiation today. Chemical methods can directly extract chromium from the ore and produce chromium carbide and chromium oxide.
Post time: Apr-30-2021